Diagnosis of
rabies in animals
Even with symptoms quite characteristic for rabies, like changes in
behaviour or difficulties in swallowing the clinical examination cannot
rule out rabies nor confirm the diagnosis. Brain tissue is the preferred
specimen for post-mortem diagnosis in both humans and animals.
Intra-vitam diagnosis is in suspect human patients is based on detecting
virus or viral RNA in saliva, neck skin biopsy or epithelial cells of
the cornea. However, due to intermittent shedding of virus and variable
diagnostic sensitivity of the methods applied, only positive results are
valid.
Detection of rabies antigen
Different
immuno-chemical methods have been developed to detect the virus or its
antigens. The most widely used method for diagnosing rabies infection in
animals and humans and recommended by both WHO and OIE is the
fluorescent antibody test (FAT). It is considered the gold standard for
rabies diagnosis. Brain tissue samples, smears or cells are treated with
antirabies serum or globulin labelled with fluorescein isothiocyanate (FITC).
Preferentially polyclonal conjugates with fluorescence-labelled
antibodies are used. Specific aggregates of rabies virus antigen are
detected by their fluorescence using a reflected light (incident light)
fluorescence microscope. The FAT is accurate, sensitive and rapid.
Results can often be obtained within 1 to 2 hours of receipt of the
specimen.
Recently a rapid immunodiagnostic test (RIDT) was developed. This simple
lateral flow test may be used under field conditions and in developing
countries with limited diagnostic resources.
Detection of rabies virus replication: inoculation tests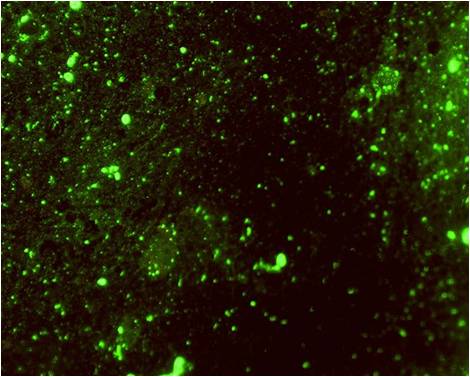
The
other group of available techniques aim at detecting the replication of
the virus on living substrates, e.g. cells or mice. Virus isolation may
be necessary to confirm the results of the FAT and for characterization
of the virus strain. Virus isolation can be performed on neuroblastoma
cells or upon intracranial inoculation of mice
In cells, rabies virus grows generally without cytopathic effect; once
again it is necessary to use FAT to confirm the presence of rabies virus
in cells. This test is as sensitive as the mouse inoculation test. Cell
culture units should be established in laboratories to replace mouse
inoculation tests as it avoids the use of life animals, is less
expensive and gives more rapid results.
In mice rabies induces clinical signs that are relatively typical but it
is to confirm with a FAT control.
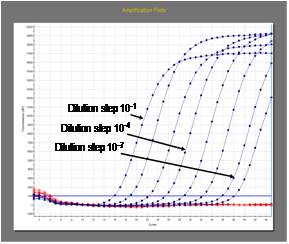
Detection of rabies virus RNA
The
reverse transcriptase (RT) polymerase chain reaction (PCR) is used to
amplify a certain fragment of the virus genome (viral RNA).
More recently, real-time PCR has been developed to increase sensitivity
and to obtain results even faster. Those techniques have the
highest level of sensitivity.
Standardization
and very stringent quality control is required. Note: this technique is
not currently recommended by WHO for routine post mortem diagnosis of
rabies but may be used as a confirmatory test or for intra vitam
diagnosis in humans. As PCR can produce false positive or false negative
results it should only be used in combination with other conventional
techniques.
Serological tests
Serological assays are not suitable for diagnosis of rabies infections
in humans and animals as virus-specific antibodies in serum tend to
appear
on average 8 days after the onset of clinical symptoms.They are
mainly used to evaluate the immune response to human and animal rabies
vaccines. The gold standard is the virus neutralisation test. Virus
neutralising antibody titres directly correspond to the level of
protection. Virus neutralisation assays are also the prescribed tests
for international trade and travel with pets. Both FAVN (Fluorescent
Antibody Neutralization Test) and RFFIT (Rapid Fluorescent Focus
Inhibition Test) are approved for determination of the titre of virus
neutralizing antibodies.